Imagine a world where all organisms' lives can be paused; their cells, tissues, and organs put into a deep sleep, all biological clocks halted. This is not science fiction, this is cryobiology.
What is cryobiology?
Cryobiology is the study of how living organisms respond to extremely low temperatures, ranging from adaptations in vertebrates to micro-organisms. It is the core process of preserving organisms and tissues by utilising cold temperatures (Kimball, 2022).
The applications of cryobiology are so diverse that there’s a multitude of its principles used in medicine, ranging from surgeries to the preservation of biological materials (Kimball, 2022). This article will focus solely on preserving biological materials and organisms.
Historical context
The history of cryobiology dates back to the 17th century, beginning with the development of the microscope, when scientist L. Spallanzani discovered that sperm could maintain mobility under cold conditions.
During the mid-19th century, Dr. James Arnott used cryoablation (All methods of destroying tissue by freezing) in the treatment of cancer, utilising salt solutions containing ice to freeze various types of cancers and shrinkage of tumours. This discovery has made a significant contribution to the development of cryobiology (Cooper, 2001).
During the mid-20th century, many scientists explored cryopreservation by experimenting with small mammals like hamsters, coming up with ways to potentially reanimate the hamsters. Through repeated attempts, scientists have discovered that microwave diathermy is the most effective method for reheating hamsters, and that hamsters frozen for as long as 50 minutes can be resuscitated, not only by microwave diathermy but also by gently rewarming the whole body under a 100W bench lamp (Smith, 1956). This in and of itself has sparked an interesting question: can humans be resuscitated through the same processes as hamsters?
Principles of cryopreservation
Cryopreservation works through a very straightforward method of freezing cells, tissues and organs until a biologically stable mode is reached with the cessation of enzymatic and metabolic activity (Karnieli, 2016). In practice, this means cooling samples to cryogenic temperatures (typically –80°C or the temperature of liquid nitrogen, –196°C), so that all cellular biochemical reactions are effectively halted. At such low temperatures, the thermal energy required for normal molecular motion and metabolism is withdrawn, “depriving biological systems of the thermal energy required for normal molecular motion and metabolism” and thus slowing cellular processes and preventing decay (Sanja, 2021). In this state, the cells are frozen and are awaiting to be thawed for future purposes. However, there are physical implications to this matter, and that term is called cryoinjury.
Cryoinjury
Cryoinjury refers to the cellular damage caused by the freezing process. This is caused by the fact that as temperature drops, water surrounding and inside the cells will begin to freeze and crystallise, causing the water crystals to destroy the cells mechanically. This makes controlling the freezing rate essential during cryopreserving biological materials.
However, controlling the freezing rate in and of itself is challenging due to the need for specificity during the process. If the freezing process happens too slowly, it’ll cause the crystallisation of water in the extracellular fluid. Causing the solute concentration outside the cells to rise sharply, this creates an osmotic gradient that draws water out of the cell, leading to dehydration. This process can damage tissues in two ways. The salt solute concentration will increase when a cell is dehydrated, causing the cell to shrink even further, causing the cell to become plasmolysed (Francesc, 2021). The ice formation surrounding the cells will also mechanically damage the tissues, squeezing the cell membrane and disrupting the extracellular structure, eventually crushing the cell completely (Sanja, 2021).
Inversely, if the freezing rate is too high, then the water present inside the cell will not have time to exit the cell, causing intercellular ice formation. This will cause damage to the cell by physically puncturing the cell membrane, resulting in a mechanically damaged cell (Heber, 1976).
Normally, the sweet spot to freeze cells to prevent this is around 1°C/min, but every cell is slightly different, as their structure can vary from plants to animals. How will we counteract this degradation of cells?
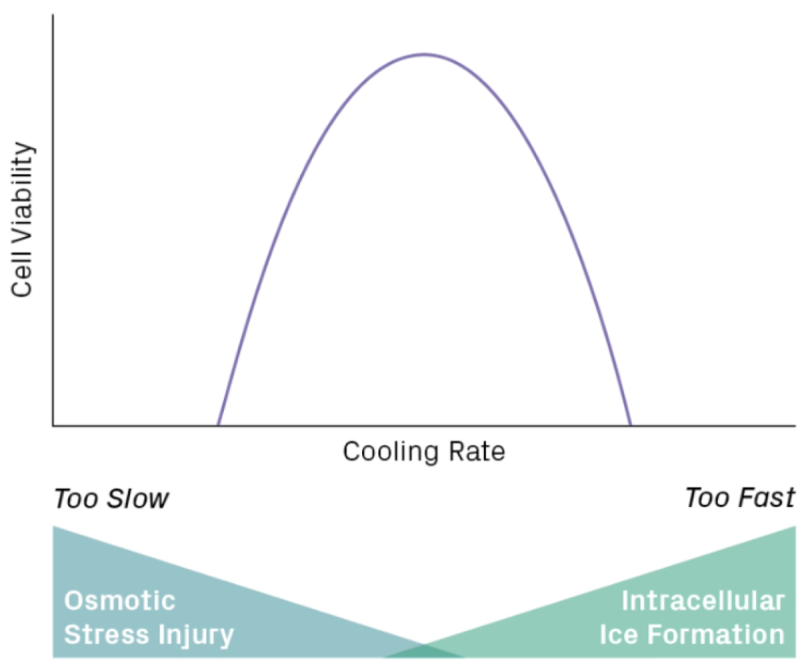
Figure 1: Effect of cooling rate on cell viability
Cryoprotectants
This is where cryoprotectants (CPAs) come in. For a cell to be cryopreserved, it is required that the cells be loaded with CPAs to preserve the intracellular structure of the cell. Examples of these protectants are glycerol, ethylene glycol, dimethyl sulfoxide, and propylene glycol. These CPAs create an osmotic gradient, causing water to move out of the cells. Then, after the initial osmotic procedure, both CPAs and water move into the cell, until an equilibrium is reached between the intracellular and extracellular spaces of the cell—or in other words, the osmotic gradient inside the cell and outside the cell is equal (Wolkers, 2018).
This can be proved through a simple experiment carried out in your residence. This experiment will help demonstrate how cryoprotectants protect biological materials during both freezing and thawing processes.
Experiment: Cryoinjury
Materials needed
- 2 Apples (Approximately the same size and weight)
- Oil
- 2 identical containers
- Freezer
- Water
Procedure
- Prepare 2 identical containers, then place one apple in each container.
- Add water to one of the dishes until the apple is submerged. Record the volume of water used.
- Add the same volume of oil to the other container.
- Place both containers into the freezer.
- Wait for 24 hours.
- Remove both containers from the freezer and wait for them to thaw completely.
- Compare the softness/texture of both apples.
Expected result
Due to the oil acting as a CPA (from the components of glycerol present in oil), it will reduce the cryoinjury affecting the apple, causing a notable difference in texture between both apples. The apple covered in oil will not be as soft as the apple that was not covered in oil.
Vitrification
An alternative strategy is vitrification. Instead of forming ice crystals, vitrification attempts to solidify the entire sample into a “glass‐like” state (Jiang Ming, 2023).
In vitrification, the solution is cooled so rapidly (and usually contains such a high CPA concentration) that water molecules do not have time to arrange into a crystal lattice. The key is to exceed the critical cooling rate for a given CPA solution so that the sample jumps past the crystallisation zone directly into a solid glass. Achieving vitrification typically requires both very high CPA concentrations (often combinations of two or more agents) and ultra-fast cooling (for example, plunging small samples into liquid nitrogen or liquid helium). In practice, cells or embryos are equilibrated in strong vitrification media – for example, exposure to solutions containing 7–15% ethylene glycol and DMSO (often with a high‑concentration sugar like 0.5 M sucrose) – and then plunged into Liquid N₂ (David, 2021).
This rapid freezing solidifies the mixture into a non-crystalline glass. If done correctly, no ice crystals form at all, avoiding the mechanical and osmotic injury of ice. One must also rewarm vitrified samples very quickly to prevent devitrification (the formation of ice during thawing). (Jiang Ming, 2023)
Vitrification has proven especially valuable for cells that are very sensitive to ice. For example, human oocytes and embryos are now routinely cryopreserved by vitrification, with post-thaw survival rates much higher than with older slow‐freeze methods. Similarly, protocols for embryonic stem cells and some other delicate cell types use vitrification to achieve recovery rates (on the order of 70–80%) that far exceed slow‐freezing outcomes (David, 2021).
In short, vitrification replaces ice with an amorphous glass, which preserves cell integrity without the fracturing forces of crystallisation. The method’s drawback is the need for very high CPA levels (which can be toxic if not properly controlled) and extremely fast cooling/warming rates. Nonetheless, by carefully optimising CPA and thermal protocols, vitrification has become a powerful cryopreservation technique. In summary, basic cryopreservation uses freezing to halt metabolism, utilising cryoprotectants to control ice and osmotic stresses, while vitrification offers an alternative that entirely bypasses ice formation through glassy solidification (Jiang Ming, 2023; Sanja, 2021).
Cryopreserving humans
In history, there was an interesting question to ponder about: if hamsters could be resuscitated through microwave diathermy, can’t humans do the same? Well, the simple answer is no, multiple attempts were commenced to try and preserve humans, turning them out to be horrific creations.
The most horrific failure was made by the storage Dewar, which was poorly designed, with uninsulated pipes leading to a series of unfortunate incidents. The bodies in the container were thawed, moved then frozen again. Then, a year later, the bodies were found to be decomposed into “a plug of fluids” at the bottom of the capsule, stuck like a child’s tongue sticking to a cold lamp post. (Michael, 1992)
Then why did they fail? Isn’t it logical to assume that if hamsters, which are living organisms, can be frozen and thawed, then can’t humans do the same? Here are a couple of reasons why.
- Lack of natural antifreeze: Humans don’t produce protective natural antifreeze, unlike frogs, which produce sugars which contain a cryoprotectant compound (glycerol) (Maddy, 2022).
- Structure of the human body: The human cell is filled with 70% water, which is the sole cause of cryoinjury, and although scientists propose the idea of vitrification, it’s needed to load with an extremely high concentration of CPAs, which could result in toxicity in the human body (Bertalan 2024).
Ethics of cryopreservation
Even if it were possible for us to cryopreserve humans, what are the arguments towards the morality issues with preservation? This raises multiple ethical questions.
- There is no guarantee that brain function and memory can be restored. How do you prove that this does not contain any form of side effects?
- What happens if cryopreservation fails due to a lack of funding or equipment malfunction over the years?
- Death is natural. Why are we finding a way to preserve humans to potentially prolong their lifespan? Shouldn’t we accept our fate and give up on immortality? (Philippa, 2016)
As these ethical considerations grow, technology will increase as well. However, hopefully, no living organisms will be sacrificed during cryobiology experiments. Additionally, sustainability must be considered, making sure that cryopreserving humans is helping us, not the opposite.
Works Cited
Chapman, Maddy. "Can Humans Survive Being Frozen Like a Popsicle?", 2022 https://www.iflscience.com/can-humans-survive-being-frozen-like-a-popsicle-65930
Chen J, Liu X, Hu Y, Chen X and Tan S (2023) Cryopreservation of tissues and organs: present, bottlenecks, and future. Front. Vet. Sci. 10:1201794. doi: 10.3389/fvets.2023.1201794
Cooper and Dawber. "The History of Cryosurgery." Journal of The Royal Society of Medicine. https://pmc.ncbi.nlm.nih.gov/articles/PMC1281398/
Francesc, F. "Researchers discover how cells survive in high salt concentrations." BioCryo, 2021. https://www.irbbarcelona.org/en/news/scientific/researchers-discover-how-cells-survive-high-salt-concentrations
Heber, U. "Water Stress During Freezing." Cryobiology, vol. 19, 1976. https://link.springer.com/chapter/10.1007/978-3-642-66429-8_16
Karnieli, O. "Stem Cell Manufacturing." Bioreactors and Downstream Processing for Stem Cell Manufacturing, 2016. https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/cryopreservation
Kimball. "Fertility and Sterility." The ART of cryopreservation and its changing landscape, 2022. https://www.sciencedirect.com/science/article/pii/S001502822200053X
Mesko, Bertalan. "Are You Going To Wake Up From Cryosleep?" Ethics Centre, 2025. https://medicalfuturist.com/are-you-going-to-wake-up-from-cryosleep/.
Michael Perry, “Suspension Failures: Lessons from the Early Years”, 1992. https://www.cryonicsarchive.org/library/suspension-failures-lessons-from-the-early-years/
Philippa, S. "The Ethics of Cryonics." The Hastings Center Report, vol. 46, no. 6, 2016, pp. 10-12. https://www.bbc.com/news/health-38031428
Sanja Bojic. "Winter is coming: the future of cryopreservation", 2021. https://bmcbiol.biomedcentral.com/articles/10.1186/s12915-021-00976-8
Smith, A., Lovelock, J. & Parkes, A. Resuscitation of Hamsters after Supercooling or Partial Crystallisation at Body Temperatures Below 0° C. Nature 173, 1136–1137 (1954). https://doi.org/10.1038/1731136a0
Whaley, David. "Cryopreservation: An Overview of Principles and Cell-Specific Considerations", Cell Transplantation. https://pmc.ncbi.nlm.nih.gov/articles/PMC7995302/
Willem F. Wolkers, “Factors Affecting the Membrane Permeability Barrier Function of Cells during Preservation Technologies”, American Chemical Society, 2018. doi.org/10.1021/acs.langmuir.8b02852
