From a young age, people are taught to dispose of waste into designated trash bins, separating different types of wastes like organic and inorganic materials for the purpose of recycling waste into reusable products. One method often talked about in schools is composting—a way to decompose organic waste by the use of microorganisms into essential nutrients that could improve soil health and overall growth of plants. Microorganisms such as bacteria and fungi that play such significant roles are frequently mentioned—but what about the tiny, wiggling earthworms that squirm in the air pockets of soil? What exactly do they do?
This article aims to explore the roles of earthworms and its ability to play a major role in vermicomposting, a type of worm-based composting. Furthermore, we will be investigating appropriate vermicomposting conditions to be implemented in our school vermicomposting program.
What is vermicomposting?
Vermicomposting is a form of composting which utilises earthworms that feed on dead organic matter. The earthworms alter the chemical and physical composition of this organic matter, which can then be used to improve soil health (Edwards et al., 2004). By breaking down organic matter, vermicomposting produces vermicompost which contains essential nutrients needed by plants and increases the availability of nutrients such as ammonium and nitrates absorbed by plants (Domínguez, 2010, pp. 53-54).
Vermicomposting consists of two different stages, the active stage and the maturation stage. During the active stage, earthworms alter the composition of organic matter by modifying the microbial activity (Lores et al. 2006), adding substances like carbohydrates and secreting substances like mucus, ammonia and urea to aid the decomposition of the organic matter (Dominguez, 2010, p. 56). The presence of microbes found in the gut of the earthworm also secrete extracellular enzymes that can break down harder substances like cellulose found in the organic matter. By moving and wiggling through the material, earthworms also add air through aeration, where it mixes the wastes altogether to enhance and further decompose the organic matter (Domínguez, 2004).
After completing the active stage, the earthworms are found in the undigested waste where it signals the start of the maturation stage. In this stage, the partially digested matter is further refined to be used for plant growth and later on in the process able to suppress plant diseases. However, the information and explanation regarding how this is reached is not yet discovered (Dominguez, 2010, p. 56).
Digestive mechanism in earthworms
Unlike the complex nature of the human digestive system, earthworms mainly have a simple digestive system consisting of a mouth, pharynx, esophagus, crop, gizzard and intestine (Edwards and Bohlen, 1996). While there are some intricate differences between different families of earthworms, we will be primarily focusing on the digestive system of Eisenia fetida—as we will use this species in our own vermicompost.
According to van Gansen (1963), Eisenia fetida has three major zones in its digestive system: the reception zone, secretory zone, and absorption zone. The reception zone consists of the mouth, pharynx and esophagus. The organic matter is first ingested by the mouth of the earthworm which passes into the pharynx. In the pharynx, an acid mucus containing enzyme amylase is produced by the pharyngeal gland to break down the organic matter. In the esophagus, calcium carbonate is secreted with mucus by a calciferous gland. Although the exact function is unclear and not yet fully understood, there are suggestions that it is used to regulate intestinal pH or to excrete excess calcium in the blood of the earthworm.
The second part, the secretory zone, consists of the crop which connects to the gizzard and the intestine of the worm. The crop stores the organic matter from the esophagus and delivers it to the gizzard (“Earthworms”). The gizzard acts as a grinder, using its muscles in its walls to crush and break down organic matter by bringing the cuticle lining of the gizzard walls together. It is then passed to the intestine where cells in intestinal walls secrete two proteases and one amylase that are enzymes that catalyse different components of the digested matter. The products then are absorbed through the intestinal epithelium where it is transported by the blood for metabolic processes or storage.
In the final absorption zone which includes the intestine and the anus, the residual matter is surrounded by a periphotic membrane of the intestine which remains surrounding the casts after excretion through the anus. Although periphotic membrane (PM) is often linked with insects, PM has been reported to work as a barrier to several toxins and harmful chemicals like insecticides, heavy metals, pathogens and other toxins (Hegedus et al, 2019). This might suggest the importance of PM in enhancing soil health and maintaining the quality of the cast excreted.
Although earthworms are often the holy grail of vermicomposting, microorganisms in organic matter play an equally important role in providing necessary nutrients to aid the organic matter passing through the digestive tract of earthworms. Moreover, some reports indicate that some microorganisms like bacteria and protozoa secrete several enzymes that act on the organic matter.
Conditions and factors
Moisture
Maintaining a suitable soil moisture content is a key factor in developing a sustainable vermicomposting system. Earthworms such as Eisenia fetida are typically composed of 75%-90% water (Grant, 1955), making them susceptible to water loss. This is primarily due to the inability of earthworms to regulate the water content in its body, thus, relying on soil moisture to maintain its water contents for survival (Kretzschmar and Bruchou, 1991) . Furthermore, the moisture content is crucial as it also affects the growth of the population of earthworms. In the case of Eisenia fetida with 80% water content, Edwards (1988) suggested a range of 75%-90% soil moisture content, varying on the organic waste used in the vermicomposting system.
According to a study by Gunadi et al. (2003) on Eisenia fetida, moisture is not significantly correlated with changes in chemical composition of the compost produced. Despite this, the same study shows that the growth in population of the earthworms is affected by moisture, showing 75% and 90% as the optimum moisture content in pig manure and cattle manure respectively. Although the chemical properties are not greatly affected, having a larger population of earthworms in the continuous process of vermicomposting is beneficial to produce more compost over time with a faster rate.
In another study conducted by N.B. Singh et al. (2004) using Perionyx Excavatus species, 40%, 50%, 60%, 70%, 80% and 90% moisture content was investigated by measuring the ash content heated up to determine the amount of organic waste decomposed and minerals broken down. From the experiment, results also showed that 70-80% was the optimum moisture content for the decomposition of vegetables. In the experiment, however, the 90% moisture content notably showed slower decomposition. It was suggested that the rate of decomposition is affected likely due to reduced oxygen availability under excessively wet conditions. This likely hindered the activity of the earthworms, as they rely on oxygen dissolving through the moist layer of their body surface (Edwards and Bohlen, 1996).
Temperature
Temperature variations in the winter season supported the life activities of Eisenia fetida more favorably than in the summer season. This can be seen from the increased organic matter degradation, nitrogen increase, and C:N ratio reductions are higher. (Garg & Gupta, 2011). Since it is nearly impossible to recreate a winter condition in a tropical climate, the vermicompost must be kept under the shade. Another study indicates that the optimum temperature for vermicomposting with the help of Eisenia fetida is between 15ºC-25ºC. (Kaplan et al, 1980). At 25ºC earthworm growth rates, organic matter decomposition, mineralisation, and nitrification increases.
pH
Eisenia fetida cannot survive in environments with a pH of below 5 and above 9. Within this range, Eisenia fetida can change the pH of the soil back to around 7. According to a study, the largest weight change is at the pH of 7. (Kaplan et al, 1980). According to Wu et al. (2009), acidic conditions (pH 3.0–5.2) inhibit the survival growth and reproduction of these worms. In a different study by Satchell et al in 1984, Eisenia fetida lost weight in moderately acidic soils (pH 3.6–4.3). This might be caused by the reduced activity and energy expenditure.
In a different study by Wu Jia Long et al. (2019), soil acidification is found to reduce growth rates and may disrupt energy budgets for metabolic costs. But as the process of vermicomposting proceeds over time, the soil pH decreases over time which might act as an inhibitor. (Biabani et al., 2018). In another study by Singh (2006) and Bhattacharya & Lane (2005), the species Perionyx excavatus performs well across a wide pH range. The reason why we use Eisenia fetida is because according to Savita et al (2025), this species decomposes organic matter faster.
Different methods in vermicomposting
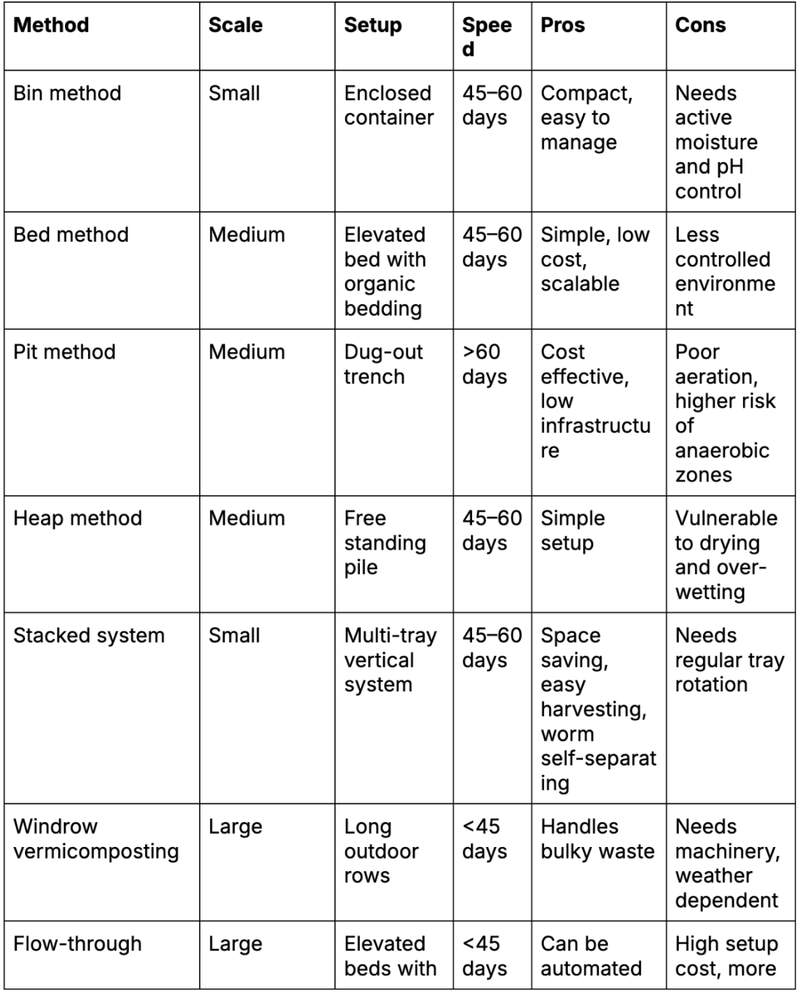
Table 1: Comparison of different methods in vermicomposting
Vermicomposting in JNY
Jakarta Nanyang School is not yet a zero-waste school. During recess periods, a significant amount of food is wasted. Some of the food waste are organic materials. These organic wastes can be placed in vermicompost bins to be converted into compost. Not only will it decrease the amount of waste being produced, but it will also create a compost that can be used around the school.
According to this article, the most suitable method is the stacked method as it is easy to maintain and relatively effective and quick. The most optimal location for the vermicompost will be the butterfly garden as it’s surrounded by trees and is relatively colder than other places in school.
Works Cited
Domínguez, J. Earthworm Ecology, edited by Soil and Water Conservation Society (U.S.) and C. A. Edwards, CRC-Press, 1998, pp. 401–424.
“Earthworms.” Penn Arts & Sciences, https://www.sas.upenn.edu/~rlenet/Earthworms.html. Accessed 21 June 2025.
Edwards, C. A., and J. Dominguez. “The influence of vermicomposts on plant growth and pest incidence.” Soil Zoology for Sustainable Development in the 21st Century, edited by N. Q. Arancon, et al., 2004, pp. 397-420.
Edwards, Clive A., et al., editors. Vermiculture Technology: Earthworms, Organic Wastes, and Environmental Management. Taylor & Francis, 2011.
Edwards, Clive A., and P.J. Bohlen. Biology and ecology of earthworms. Springer Netherlands, 1996.
Edwards, Clive Arthur, et al., editors. Earthworms in Waste and Environmental Management. SPB Academic Publishing, 1988.
Gansen, P. van, and Société royale zoologique de Belgique. “Annales de la Société royale zoologique de Belgique.” Structure and functions of the digestive canal of the earthworm Eisenia foetida Savigny., 1963, pp. 1-120, https://www.biodiversitylibrary.org/bibliography/16350.
Grant, W. C. “Studies on Moisture Relationships in Earthworm.” Ecology, vol. 36, no. 3, 1955, pp. 400-407, https://www.jstor.org/stable/1929574.
Gunadi, B., et al. “The influence of different moisture levels on the growth, fecundity and survival of Eisenia fetida (Savigny) in cattle and pig manure solids.” European Journal of Soil Biology, vol. 39, no. 1, 2003, pp. 19-24, https://www.sciencedirect.com/science/article/abs/pii/S1164556302000055.
Hegedus, D. D., et al. “Peritrophic matrix formation.” Journal of Insect Physiology, vol. 117, 2019, https://www.sciencedirect.com/science/article/abs/pii/S0022191018304761.
Kretzschmar, A., and C. Bruchou. “Weight response to the soil water potential of the earthworm Aporrectodea longa.” Biology and Fertility of Soils, vol. 12, 1991, pp. 209–212, https://link.springer.com/article/10.1007/BF00337204.
Lores, M., et al. “Using FAME profiles for the characterization of animal wastes and vermicomposts.” Soil Biology and Biochemistry, vol. 38, no. 9, 2016, pp. 2993-2996, https://www.sciencedirect.com/science/article/abs/pii/S0038071706002446.
Pilli, Kiran & Sridhar, Durgam. (2019). Vermicomposting and its uses in Sustainable Agriculture.
Singh, N. B., et al. “Optimum Moisture Requirement during Vermicomposting Using Perionyx excavatus.” Applied Ecology and Environmental Research, vol. 2, no. 1, 2004, pp. 53-62, https://www.aloki.hu/pdf/02053062.pdf.
